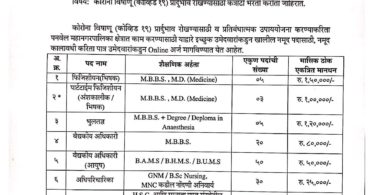
Applications are invited for the following posts for recruitment on purely temporary and contractual basis under The Biotechnology industry Research Assistance Council (BIRAC) funded project titled *To establish a ready network of clinical tria! umits across the National Cancer Grid to prozzzote multi-centrie collaborative research io the field of drug and device development”. This study aims to set up s research lab to conduct Clinical Trials across a spectrum of cancers.
|
N |
Position |
No Ot Posit |
Minimum Qualification |
Exp |
Age Role In The Project Limit |
Monthly Salary |
|
1 |
Clinical Trial Coordinator |
2 |
BAMS/BDS/ BHMS/MSC
with Post Graduate in clinical research or health or life sciences |
2 to Years |
Overseeing the smooth running of clinical trials. Collecting, coding and analyzing data obtained from
28 research. ears Monitoring research participants to ensure adherence to study rules. Adhering to research regulatory standards. |
30000 |
Resea rch Nurse 2 BSc Nursing
B.Sc.;
Computer
Ensures compliance with each study’s protocol by providing thorough review and documentation at each subject study visit
Participates in recruitment and selection of study participants by interviewing and documenting medical history to determine compliance with eligibility requirements Performs medical tests, including, but not limited to, vital signs, imaging studies, and
2 to 28 electrocardiograms years Administers investigational medications and performs patient assessments during clinic visits to
determine presence of side effects; notifies Principle Investigator of findings/issues
Provides patient education and medical information to study patients to ensure understanding of proper medication dosage, administration, and disease treatment Documents medical data in patient chart to capture protocol requirements
6mo
15000
Data Entry operator
skills(MS Office, others) Desirable: Statistical Software,
Clinical triaI/ registry data entry, report preparation, database maintenance
15000